-
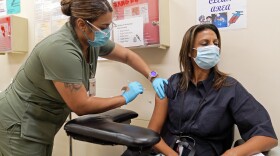
Vaccines for a fall immunization drive against COVID-19 just got the green light from the Food and Drug Administration. The agency says the vaccines can protect people, as hospitalizations tick up.
-
From convenience stores to online, the tablet "will be an available option for millions of people in the United States," the director of the FDA's Center for Drug Evaluation and Research says.
-
The bivalent vaccines offer protection against both the original strain of COVID and the omicron variants. The updated recommendations aim to simplify the vaccination schedule in the U.S.
-
A federal judge in Texas stayed the FDA's approval of the drug mifepristone, while a federal judge in Washington state blocked any FDA change in access.
-
The new approach would simplify vaccination guidance so that, every fall, people would get a new shot, updated to try to match whatever variant is dominant.
-
The FDA has confirmed the nation is experiencing a shortage of Adderall after many pharmacies around the country have been unable to fill prescriptions and keep up with demand.
-
CDC Director Rochelle Walensky has signed off on updated versions of the Moderna and Pfizer-BioNTech vaccines that target the original virus and the omicron subvariants.
-
The new shots from Moderna and Pfizer-BioNTech target both the original strain of the coronavirus and the omicron BA.4/BA.5 subvariants that most people are catching now.
-
The Food and Drug Administration is planning to authorize a new generation of COVID-19 boosters this week that for the first time will target the omicron variant.
-
Pfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
© 2026 KUT Public Media
A service of the Moody College of Communication at the University of Texas at Austin
webmaster@kutx.org
A service of the Moody College of Communication at the University of Texas at Austin
webmaster@kutx.org
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations